Have you ever wondered why, in most species, males are larger and more ornamented than females? It’s an evolutionarily determined aspect of biology, but what does it mean for human health and disease? What are the implications of needing one chart to describe normal growth in boys, and another to describe normal growth in girls? Why are there two normals for growth, and does it matter for a disease of growth like cancer?
I’m a pediatric brain tumor doctor and scientist and am interested in developing new treatments for glioblastoma (GBM) and other malignant brain tumors. Glioblastoma is the most common malignant brain tumor and killed the late Sens. John McCain and Ted Kennedy, and Beau Biden III, the eldest child of former U.S. Vice President Joe Biden.
In this new year, about 22,000 Americans will develop glioblastoma, and nearly the same number will die from it. While GBM occurs in both males and females, we can reliably predict that of the 22,000 new cases, 8,500 will be in females while the remaining 13,500 cases will be in males. Moreover, the female GBM patients can be expected to survive about six months longer than the male patients, on average.
My colleagues and I wondered whether basic differences in biology might explain why males were more vulnerable to these malignant brain tumors and why their survival time was shorter than for females. We hypothesized that if there were differences between the male and female version of glioblastoma, we might be able to generate new, sex-specific approaches to treatment that would improve outcomes for everyone.
Sex and disease
Many human diseases exhibit substantial sex differences in their frequency and severity.Autoimmune disorders such as systemic lupus erythematosus occur nine times more frequently in females than males, and psychiatric diseases such as like depression occur nearly twice as frequently in females compared to males. The implications of sex differences in cancer have not been extensively investigated in clinical or laboratory research.
While there is a great interest in developing more personalized approaches to cancer treatment, a patient’s sex, a key feature of personalization, has not yet been incorporated into this paradigm. In our recent study in Science Translational Medicine, my collaborators and I provide what we think is compelling evidence that patients’ sex should be incorporated into treatments for glioblastoma and more thoroughly investigated in the laboratory.
In our study, we sought to determine whether differences in survival for males and females with glioblastoma were a consequence of different responses to the standard treatment; surgery, radiation and temozolomide chemotherapy. And, if there were, we wanted to explore whether there were sex-specific mechanisms that contribute to treatment response and survival in males and females.
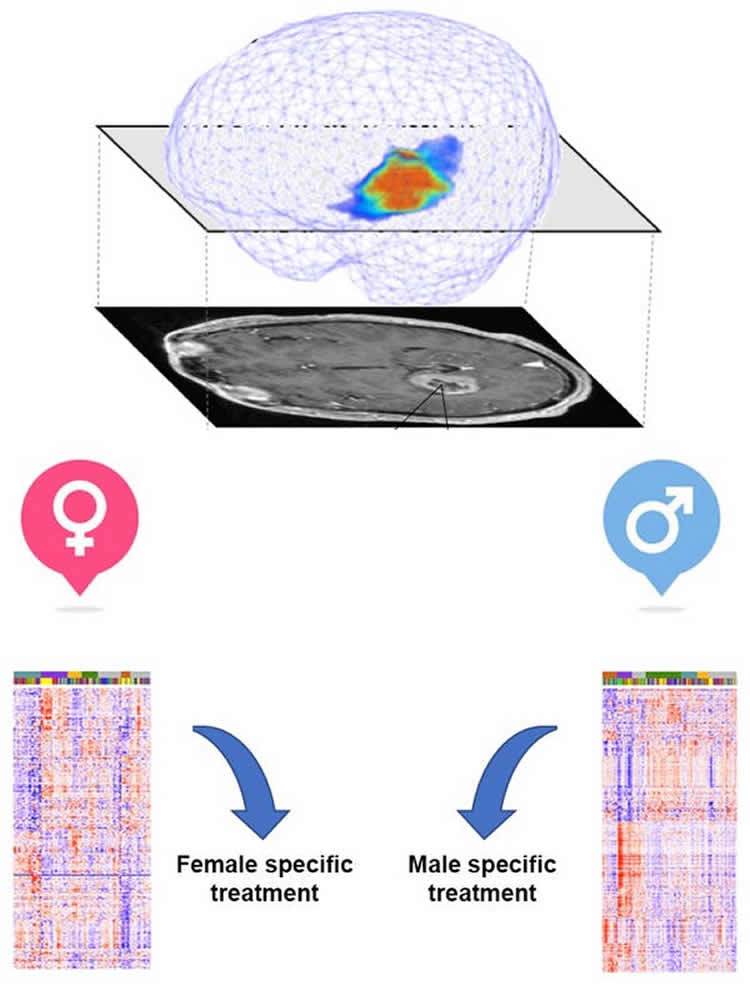
First, we analyzed standard magnetic resonance images – or MRIs – of 371 patients’ brains taken during routine treatment at the Mayo Clinic. We measured how the tumor proliferated and grew in the brains of these patients and how the tumor invaded and spread into the surrounding brain tissue. Both proliferation and invasion ultimately kill the patient.
We found that in female patients, radiation and chemotherapy treatment slowed tumor proliferation, but this was not the case for male patients. Male tumors continued to grow at the same rate, unhindered by these treatments. In addition, we found that tumor proliferation predicted survival for both males and females but invasion only affected survival for females.
How genes affect cancer growth
We concluded that female patients’ better response to standard treatment for glioblastoma and better survival might be determined in a sex-specific fashion by invasion in addition to proliferation. However, survival in male patients appeared to only be influenced by proliferation.
We next sought to identify what causes these differences. Among the ways we have to understand cancer biology is to look at the differences between the genes that cancer cells use to grow and respond to radiation and chemotherapy. We can then compare these genes to those that normal cells use.
The genes are the tools that cells use for these functions. If researchers can identify the tools cancers use to grow, we can try to design treatments to disable them. To do this, we took advantage of a large amount of publicly available data through The Cancer Genome Atlas, the Rembrandt study and two additional databases on cancer gene activity, which geneticists call refer to as gene expression. Then using a specialized kind of math, known as Joint and Individual Variance Explained, we found significant differences in the activities of genes in male and female glioblastoma.
We think it is important that we discovered some genes had different effects on survival in male and female patients. For instance, when the levels of a gene called CCNB2 were low in males, they survived longer. This was not the case for females. In females, when levels of a gene known as PCDHB were low, females survived longer. This was not the case for males. This suggests that it is essential that researchers study the impact of drugs on male and female cells separately, for GBM and possibly other cancers.
We were intrigued that male survival was significantly determined by genes that controlled rates of cell division, whereas female survival was significantly determined by genes that can regulate the ability of a cancer cell to migrate to a different area of the brain. This suggests that some types of drugs that target how cancer cells divide might work better in males, whereas drugs that inhibit cancer cells from spreading to distant organs might be more effective in females.
What drives cancers in men versus women?
Finally, we used asked whether the levels of gene expression mattered for how the cancer cells respond to chemotherapy in a dish. This is important because it might help researchers, including our team, to design treatments for patients by screening large numbers of drugs to find the ones to which they are most sensitive.
We found that the low levels of genes involved in proliferation were linked to longer survival in male patients and greater sensitivity to chemotherapy in a dish. Similarly, we found that low levels of genes involved in cell migration were associated with longer survival in female patients and increased sensitivity to the same chemotherapy in a dish.
Together, these results suggest that it may be possible to improve outcomes for all glioblastoma patients, and possibly other cancers, by using sex-based approaches to diagnosis and treatment. That’s because many cancers are more common in males and it is possible that for each of these cancers sex-specific approaches would be beneficial.
We believe this should be evaluated in prospective clinical trials of standard and novel therapeutics. We have just begun a clinical trial in which we are gathering data about sex differences in metabolism and response to a ketogenic diet in which tumors are starved of glucose in children with relapsed brain tumors. We are also actively working to determine when, during normal development, sex differences in risk for cancer and sensitivity to treatment arise.